Electron-Rich Phenothiazine Congeners and Beyond: Synthesis and Electronic Properties of Isomeric Dithieno[1,4]thiazines.
L. May, T. J. J. Müller, Chem. Eur. J. 2020, 26, 12111–12118; doi.org/10.1002/chem.202000137.
Abstract
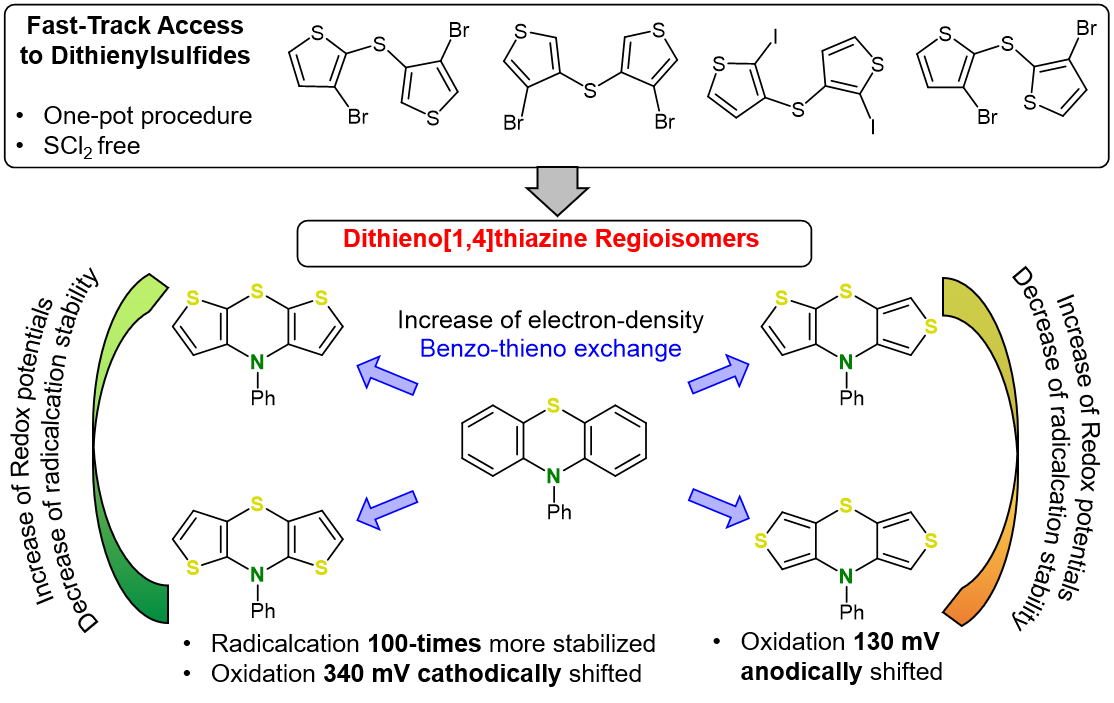
A series of isomeric dithieno[1,4]thiazines is accessible via intermolecular-intramolecular Buchwald-Hartwig amination starting from dihalodithienyl sulfides. The electronic properties of dithieno[1,4]thiazine isomers differ conspicuously over a broad range depending on the thiophene-thiazine anellation: a large cathodic (340 mV) or an anodic shift (130 mV) of the redox potentials relative to corresponding phenothiazines is possible. Structure-property relationships of the dithieno[1,4]thiazine constitution derived from DFT-calculations and cyclic voltammetry not only reveal increased electron density but also different delocalization of the radical cations that determines the electrochemical properties significantly. In addition, photophysical properties (absorption and emission) qualify dithieno[1,4]thiazines as promising substitutes of phenothiazine and beyond due to increased tunable electron richness.